AMERICA (Enmaeya News) - October 29, 2025
The Food and Drug Administration (FDA) has approved a new once-daily pill that works without hormones to treat moderate to severe hot flashes and night sweats (vasomotor symptoms) caused by menopause.
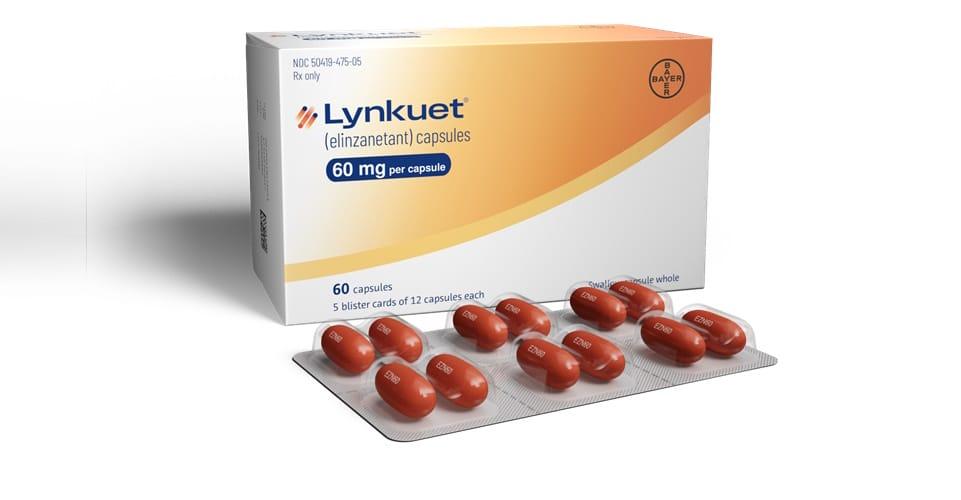
The drug, elinzanetant (brand name Lynkuet), developed by Bayer, is expected to become available in the U.S. in November.
Elinzanetant acts on two distinct brain receptors involved in temperature regulation, setting it apart from prior non-hormonal treatments that targeted only one receptor.
In a Phase III clinical trial involving 628 post-menopausal women over 12 weeks, those taking the drug saw over a 73% reduction in hot-flash frequency, compared with a 47% reduction in the placebo group, according to Bayer.
For many women, especially those who cannot or choose not to use hormone replacement therapy (HRT), hot flashes can be debilitating, affecting sleep, daily functioning, and quality of life. This approval provides an important alternative.
The FDA approval of Lynkuet marks a significant step forward in offering non-hormonal options for managing menopause symptoms, giving women more choices to improve comfort and daily quality of life.