AMERICA (Enmaeya News) - December 25, 2025
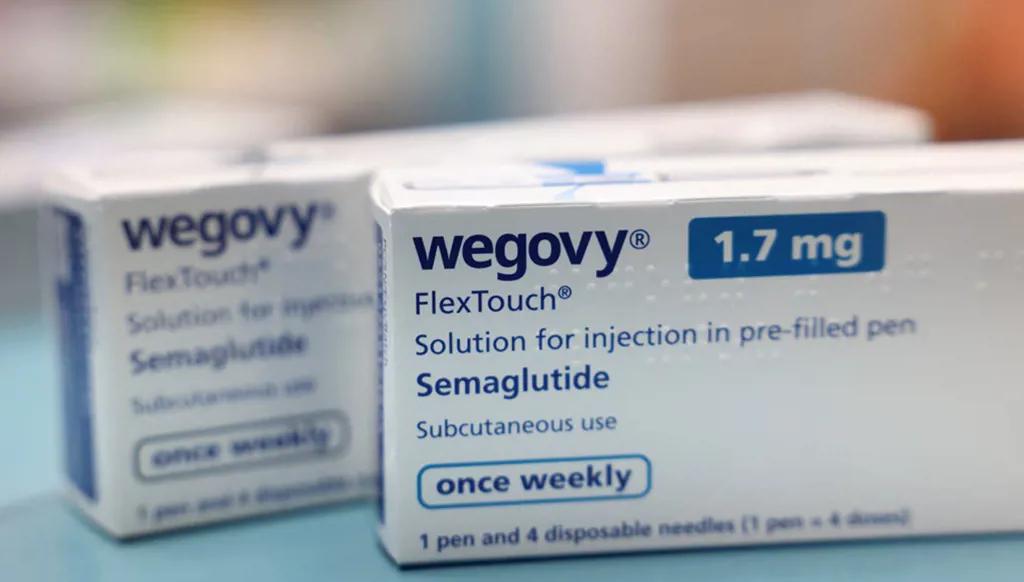
The U.S. Food and Drug Administration (FDA) has approved the first daily oral version of the popular obesity drug Wegovy, marking a major step in expanding access to weight-loss medications amid the high costs of existing injectable treatments.
The approval, announced December 22, gives Novo Nordisk an advantage in the race to introduce the first oral obesity pill in the U.S.
Like the widely used injections, the new pill works through the GLP-1 mechanism, mimicking natural hormones that regulate appetite and increase satiety.
In recent years, injectable treatments such as Wegovy and Eli Lilly’s Zepboundhave transformed obesity management, a condition affecting nearly 100 million Americans. Novo Nordisk said the pill will be available in pharmacies within weeks.
The new pill contains 25 mg of semaglutide, the same active ingredient in Wegovy injections and Ozempic, as well as the previously approved oral diabetes drug Rybelsus.
Clinical trials showed participants taking the pill lost an average of 13.6% of their body weight over 15 months, compared with 2.2% for the placebo group, closely matching the 15% average weight loss achieved with injections.
Patients must take the pill on an empty stomach with water, waiting 30 minutes before eating or drinking. Novo Nordisk said initial pricing will start at $149 per month for some providers, lower than injections that can exceed $1,000 monthly.
The FDA’s approval of the daily oral Wegovy pill marks a potential turning point in obesity treatment, making effective weight-loss medication more accessible and manageable for millions of Americans.